By Dr Abha Malik and Dr Bryn Atmore
Introduction
Therapeutic radiation has been a long-known treatment for cancer, however, the side effects are the major limitation.
The gastrointestinal (GI) side effects are more common, particularly as the bowel is more vulnerable because of its anatomical location and rapidity of turnover of many GI epithelial cell types.
Severity of radiation damage depends also on patient factors–chemotherapy, radiotherapy regimen and organ mobility.
Radiation damage can be seen as mucosal ulceration, inflammatory infiltrate, epithelial atypia and fibrosis. These changes can be sometimes confused with viral inclusions or inflammatory bowel disease. Detailed history immunohistochemistry (IHC) can help in a confident diagnosis.
|
"Radiation damage can be seen as |
Clinical history
Patient with a history of unknown primary, with documented bony metastasis, presented with gastric ulcer (which looked benign according to the clinician).
Specimen sent from gastric antrum and gastric ulcer to rule out malignancy.
Macroscopic findings
- Specimen labelled as gastric ulcer biopsy.
- Specimen consisted of six pieces of pale tissue measuring 3mm to 4mm in maximum dimension.
Microscopic findings
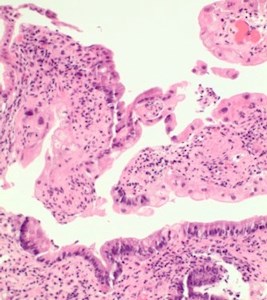
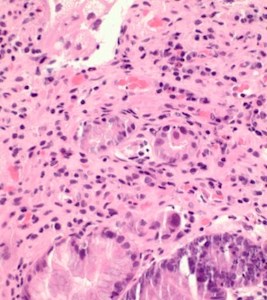
- Sections revealed gastric mucosa with foveolar type glands, and active chronic inflammation in lamina propria.
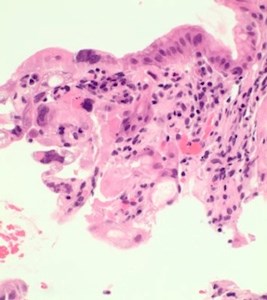
- In focal areas, the crypts and the glands show some reactive changes with cytoplasmic vacuolation, nuclear enlargement and nuclear irregularities with some nuclear pseudoinclusions. Few crypts show degeneration (Figs 2,3).
- Occasional nuclei show dense eosinophilic homogenisation of chromatin (Figs 3,4). Few bizarre nuclei with cytoplasmic vacuolization were noted. The nuclear to cytoplasmic ratio was low. These changes merge with normal gastric pits.
- Stromal fibroblasts also appeared atypical, and some vessels show thickening of walls.
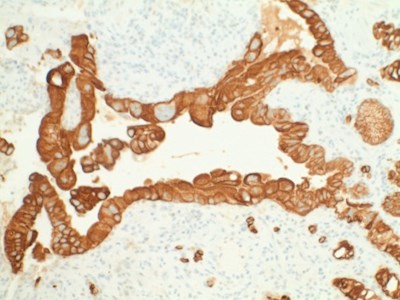
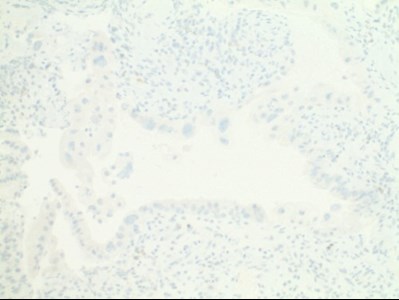
- Atypical cells were positive for AE/AE3 and negative for cytomegalovirus (CMV) and herpes simplex virus (HSV) immunostaining (Figs 5,6,7).
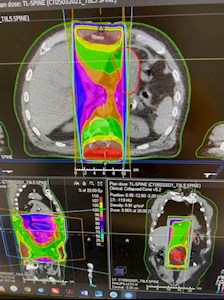
- Case work-up was done and radiation oncologist confirmed that medial part of the stomach was caught between radiation field (Fig 1).
- Clinical history, histological findings and IHC correlated with the final diagnosis.
|
|
|
|
Figure 1. CT abdomen – stomach |
Figure 2. Ulceration of gastric mucosa, with |
|
|
|
|
Figure 3. The surface epithelium and crypts |
Figure 4. Atypical cells showing multinucleation |
|
|
|
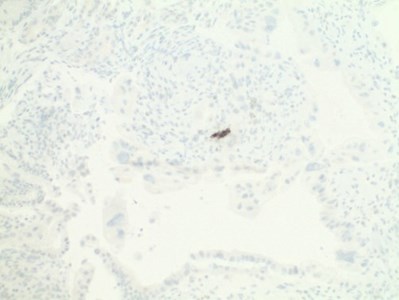 |
| Figures 5,6,7. Immunohistochemistry: Atypical cells were positive for AE/AE3 and negative for CMV, HSV immunostaining. |
||
Discussion
This case highlights the importance of epithelial atypia secondary to radiotherapy, which may be mistaken for neoplastic or preneoplastic conditions and is a diagnostic pitfall.
Both chemotherapy and radiotherapy are increasingly utilised treatment modalities. Patients can present with various symptoms and findings on endoscopy etc. It’s important to be aware of this complication, share relevant information with the diagnosticians to avoid misdiagnoses and over-treatment.
References
- Feakin, R.M. (2020). Radiation and the gastrointestinal tract, in R.M. Feakin (ed.), Non-Neoplastic pathology of the gastrointestinal tract: A practical guide to biopsy diagnosis (pp. 30–51). Cambridge: Cambridge University Press
- Brien, T.P. Gastric Dysplasia-like epithelia atypia associated with chemoradiotherapy for oesophageal cancer: clinicopathologic and immunohistochemical study of 15 cases. Mod Pathol. 2001;14(5):389–396
- Weidner, N., Smith, J.G. Peptic ulceration with marked epithelia atypia following hepatic arterial infusion chemotherapy. A lesion initially misinterpreted as carcinoma. Am J Surg Pathol. 1983 Apr;7(3):261–8
- Andreyev, H.J. Gastrointestinal problems following pelvic radiotherapy: the past, the present and the future. Clin Oncol (RColl Radiol); 2007;19:790–799.
- Kennedy, G.D, Heise, C.P. Radiation colitis and proctitis. Clin Colon Rectal Surg. 2007;20:64–72
About the authors:

Dr Abha Malik
MBBS MD FRCPA
Lab: Northern Beaches Hospital
Speciality: Anatomical pathology
Areas of Interest: Thoracic, gastrointestinal, hepatobiliary, lymph node pathology, cytology, head & neck, digital pathology, quality management
Phone: (02) 8329 2858
Email: abha.malik1@clinicallabs.com.au
Dr Abha Malik is a widely experienced anatomical pathologist with broad-based expertise, specialising in GIT, lung, breast and hepatobiliary pathology, having worked in some of the largest labs in Australia, North America, Europe and India. She is passionate about quality, patient safety and informatics.

Dr Bryn Atmore
MBBS FRCPA
Lab: Northern Beaches Hospital
Speciality: Anatomical pathology, cytopathology
Areas of Interest: Gastrointestinal, cardiorespiratory, breast, endocrine, and genitourinary pathology, and cytopathology
Phone: 1300 134 111
Email: bryn.atmore@clinicallabs.com.au
Dr Bryn Atmore joined Clinical Labs, NSW as Deputy Directory Pathology Northern Beaches Hospital. He is the onsite senior diagnostic consultant at the 500-bed hospital, and is a clinical lecturer with the University of Sydney. Dr Atmore brings 15 years of broad clinical and academic experience and specialises in multiple histopathology areas, including gastrointestinal, cardiorespiratory, breast, endocrine, and genitourinary pathology, as well as cytopathology.