
Liquid Biopsy: The Revolutionary Aspect of Molecular Oncology
Dr Mirette Saad
Cancer, a leading cause of mortality, is associated with mutated genes. Analysis of tumour associated genetic alterations is increasingly used for diagnostic, prognostic and treatment purposes. Precision or personalised medicine harnesses genomic knowledge banks to tailor individualised treatments based on patients’ or their tumours’ genetic signatures.
Somatic Mutations in Cancer
A high-quality genomic analysis is critical for personalised pharmacotherapy in patients with cancer. Identifying activated therapeutic gene targets (e.g., BRAF in melanoma, EGFR in lung cancer and KRAS in colorectal cancer) with established cancer associations, using focused panels for targeted cancer sequencing, allows for deeper coverage of those genes and higher sensitivity to confidently call rare variants (e.g., PTEN and KIT) in rare tumour subclones, including FFPE tissue. The advent of therapies targeting genomic alterations has improved the care of patients with certain types of cancer dramatically. Thus, elucidating the genetic profile of a given tumour is potentially useful in designing tailored treatment regimens that avoid unnecessary toxic therapy.
Liquid Biopsy
The genetic profile of solid tumours is currently obtained from surgical or biopsy specimens; however, performing biopsies, particularly in lung cancer, is not always possible in advanced disease stages and is associated with potential complications. Also, information acquired from a single biopsy provides a limited snap-shot of a tumour and might fail to reflect its true genetic and cellular heterogeneity.
While molecular targets were initially detected in nucleic acid samples extracted from tumour solid tissues, detection of circulating nucleic acids in blood has enabled the development of what has become known as “liquid biopsy”.
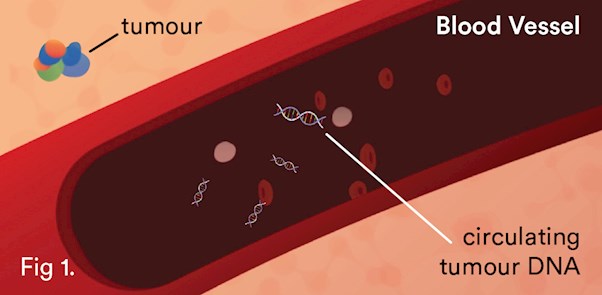
Certain fragments of DNA, cell-free DNA (cfDNA), have been shown to be elevated in the plasma of patients with cancer. This increase is the result of the rapid turnover of cells within the tumour, releasing DNA into the circulation1 (see Fig. 1). With new highly sensitive technologies, cell-free circulating DNA can now be isolated and analysed. cfDNA analysis can complement and in many instances correlate to solid tissue biopsies3,4 for real-time molecular monitoring of treatment, detection of recurrence, and tracking resistance. Circulating tumour DNA (ctDNA) reflects the overall tumour information and is not biased by analysing only a small fraction of the tumour. It is always accessible, in contrast to the lung cancer tissue.
Various studies in breast, lung and colorectal cancers have demonstrated the potential application of ctDNA analysis at each stage of clinical management: early diagnosis12,13, molecular profiling14, prognostication12,15,16, detection of residual disease7,17, monitoring response and clonal evolution18,19,20. Lastly, there have been recent approvals by the Federal Drug Administration (FDA) and the European Medicines Agency (EMA) for detection of the tyrosine kinase resistant clone EGFR p.T790M mutation in plasma as a companion diagnostic for second-line treatment of metastatic EGFR-mutant non-small cell lung cancer (NSCLC)21, 25.
Prospective research cohort studies are ongoing to explore the possible clinical applications of ctDNA in cancer. The inclusion of ctDNA analysis into multiple clinical trials (ClinicalTrials.gov), signals the recent integration of this form of biomarker into routine clinical oncology.
Implementation of Aspect Liquid Biopsy
Clinical Labs has analytically validated an integrated test process for a comprehensive mutation profiling assay for clinical oncology patients using circulating tumour derived DNA extracted from blood.
Our technology, including (NGS), MassArray Agena Biosciences ULTRASeek and Droplet Digital PCR (ddPCR), is able to identify clinically relevant variants at a sensitivity down to 0.5% or less.
Aspect Liquid Biopsy is Less Invasive
Surgical tissue biopsies have some inherent shortcomings and risks, and do not guarantee enough material will be collected for accurate analysis in clinical practice. Liquid biopsy provides a noninvasive alternative sample source, allowing the identification of genomic alterations that can be addressed by targeted therapy. This non-invasive type of liquid biopsy can be taken easily and repeatedly over the course of a patient’s treatment. ctDNA provides new insight into diagnosis, prognosis and patient followup compared to traditional tissue biopsy.
The Quality Choice for Cancer Treatment Response Monitoring
Early detection is the holy grail of cancer management. The biggest advantage of liquid biopsy is the ability to detect the cancer biomarkers in blood earlier than conventional methods22,23. While correlating with imaging scans, it has been demonstrated that monitoring for tumour-derived DNA in the plasma can identify relapse or drug resistance well before clinical signs and symptoms appear, enabling earlier intervention and better outcomes22,23.
In the future, instead of extensive imaging and invasive tissue biopsies, employing ctDNA as liquid biopsies could be used to guide cancer treatment decisions and perhaps even screen for tumours that are not yet visible on imaging.
Over the past several years there have been multiple studies demonstrating the clinical utility of liquid biopsy ctDNA analysis following surgical resection of colorectal cancers (CRCs)7. Most studies demonstrate better outcomes when no tumour-derived DNA is found in patients following surgery, or chemotherapy in colorectal cancer patients7, whereas those where tumour DNA is still present do better with the addition of more aggressive targeted treatment or chemotherapy.
It is important to note that for melanoma patients who harbour a BRAF mutation, targeted therapy remains the first-line treatment, particularly in Australia11. Tracking of BRAFv600 ctDNA levels as patients undergo targeted treatment can potentially help identify the period when resistance to targeted therapy emerges. Gray et al. and Lee et al.22,24 showed that baseline ctDNA levels predict response to immunotherapy in melanoma patients, and low basal ctDNA levels were significantly associated with long-term clinical benefit. Nevertheless, prospective clinical studies of survival in a larger cohort of patients are underway to validate the predictive value of ctDNA in melanoma patients treated with immunotherapy.
Aspect Liquid Biopsy Can Guide Treatment Decisions
Liquid biopsy can offer valuable insights into how best to treat cancer. ctDNA can be used to monitor the effects of cancer treatment and give an early warning about possible recurrence. The detection of resistant clones can offer clues to the reasons for treatment resistance5,6 (see Fig.2). Recently, a third-generation EGFR Tyrosine Kinase Inhibitor (TKI), which is effective in tumours harbouring the T790M EGFR mutation (~50-60% of lung cancer patients5,6,10), was approved in Australia for patients with NSCLC harbouring the EGFR T790M mutation following progression on an EGFR TKI9.
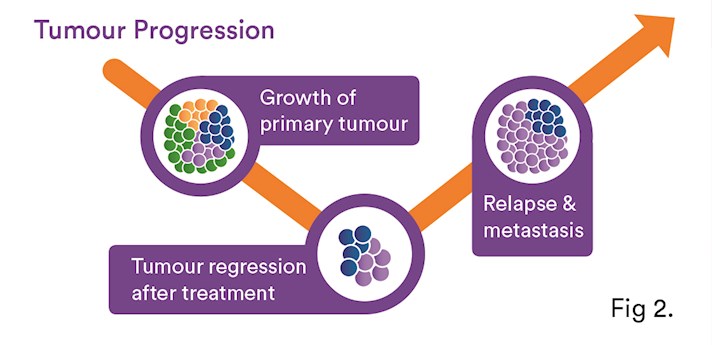
Serial analysis of ctDNA from the time of diagnosis throughout treatment can provide a dynamic picture of molecular disease changes, providing evidence that this non-invasive approach could also be used to monitor the development of secondary resistance and identify heterogeneous sub-clonal populations of tumour cells developing during the course of treatment. NCCN guidelines8 recommend the use of liquid biopsy for lung cancer patients as an alternative for tissue in initial T790M EGFR testing2,6. However, if the plasma is negative, then a tissue biopsy is recommended, if feasible.
About the Author: Dr Mirette Saad

Chemical Pathologist, Molecular Genetic Pathology
National Clinical Director of Molecular Pathology
MBBS (HONS), MD (HONS), MAACB, FRCPA, PHD
Email: mirette.saad@clinicallabs.com.au
Phone: (03) 9538 6762
Location: Victoria, Clayton
Precision Medicine, Cancer Genetics, Antenatal Screening, NIPT, Endocrine, Fertility Testing and Research & Medical Teaching
Dr Mirette Saad is a Consultant Chemical Pathologist and the National Clinical Director of Molecular Genetic Pathology at Australian Clinical Labs. At Clinical Labs, Dr Mirette Saad leads the Molecular Genetic testing for Non-Invasive Prenatal Testing (NIPT), carrier screening, personalised drug therapy and cancer. She is a member of the RCPA Chemical Pathology Advisory Committee and a Chair of precision medicine services at Australian Clinical Labs.
How to Order?
Health Practitioners can order Aspect Liquid Biopsy for cancer patients using the Aspect request form. Some required information is listed on the form, including type of cancer and whether it is a new diagnosis and if the patient is on anti-tumour therapy (if known). Health Practitioners can download the Aspect Liquid Biopsy form below.
What is the cost?
Out-of-pocket fee of $550. (No Medicare rebate available)
When will results be available?
Results will be available after 5-7 business days from the sample receipt date.
How is it collected?
This test requires two 10ml blood samples which can be taken at any of our
collection centres.
References
1. Fleischhacker M et al., (2007) Biochem Biophys Acta 1775:181-232.
2. John T et al., (2017) Asia-Pacific J of Clin Oncol 13:296-303.
3. Thierry A et al., (2014) Nat Med 20:430-435.
4. Newman A. M et al., (2014) Nat Med 20 (5):548-554.
5. Thress K et al., (2015) Lung Cancer 90:509-515.
6. Wu Y et al., (2017) Br J cancer 116:175-185.
7. Tie J et al., (2016) Sci Transl Med 8:346ra92.
8. National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology: Non-small cell lung cancer (7 April 2017)
9. AstraZeneca. TAGRISSO (osimertinib mesilate) product information; 2016.
10. Yu H et al., (2013) Clin Cancer Res 19:2240–2247.
11. Spagnolo F et al., (2015) Onco Targets Ther 8:157-168.
12. Bettegowda C et al., (2014) Sci Transl Med 6:224ra24.
13. Haselmann V et al., (2018) Clinical Chemistry 64 (5):830–842
14. Murtaza M et al., (2015) Nat Commun 6:8760.
15. Dawson S et al., (2013) N Engl J Med 368 (13):1199-1209.
16. Forshew T et al., (2012) Sci Transl Med 4 (136):136ra68-136ra68.
17. Garcia-Murillas I et al., (2015) Sci Transl Med 7 (302):302ra133-302ra133.
18. Murtaza M et al., (2013) Nature 497 (7447):108-112.
19. Wang W et al., (2017) Cancer Med 6 (1):154-162.
20. Van Emburgh B et al., (2014) Mol Oncol 8 (6):1084-1094.
21. Premarket approval P150044 d Cobas EGFR MUTATION TEST V2. Cited 2016; Available from: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id%C2%BCP150044
22. Gray E et al., (2015) Oncotarget 6 (39):42008-42018.
23. Girotti M et al., (2016) Cancer Discov 6 (3):286-299.
24. Lee J et al., (2017), Ann Oncol.
25. Calapre L et al., (2017) Cancer Letters 404:62-69.






