Written by Dr Jenny Grew
Australia and New Zealand have the highest incidence of melanoma in the world. Atypical pigmented skin lesions are therefore a common clinical presentation. This article aims to highlight some of the important recommendations from two recent publications, the Cancer Council guidelines for the diagnosis and management of melanoma (wiki.cancer.org.au)* and the WHO Classification of Skin Tumours (2018).
*First update since 2008, now in electronic “wiki” format for ease of updates as new evidence becomes available in this era of rapid advances.
Melanoma is a malignancy of melanocytes, the pigmentproducing cells of the skin and mucosa. Most cutaneous melanomas are caused by mutations and molecular events as a result of ultraviolet irradiation.
Melanoma continues to be a significant public health concern in Australia, responsible for significant morbidity and mortality.
There are 13,000 new cases of melanoma and over 1,750 deaths each year. It is the commonest cause of cancer and cancer deaths in young adults and the third most common cancer in older adults.
CLASSIFICATION OF MELANOMA
Melanoma subtypes differ depending on whether they occur on intermittently or chronically sun-exposed skin or in sun-shielded sites.
Melanomas arising in sun-exposed skin
Low degree of cumulative sun damage (CSD)
• Superficial spreading melanoma
• Nodular melanoma subset
High degree of cumulative sun damage
• Lentigo maligna
• Nodular melanoma subset
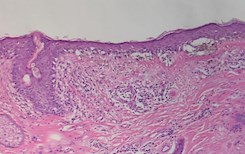
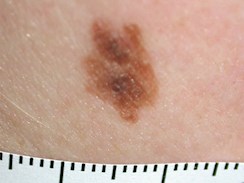
Desmoplastic melanoma
|
|
|
|
Desmoplastic melanoma – clinical appearance. |
Desmoplastic melanoma – histology. Note spindle cells |
Melanomas arising at sun-shielded sites or without known aetiological associations with UV radiation exposure include acral, mucosal and uveal types and those arising in congenital naevi and blue naevi.
MAKING THE DIAGNOSIS - WHAT TYPE OF BIOPSY?
Many different pigmented skin lesions may be encountered in general and specialist dermatology practice.
For example:
- Increased melanin pigmentation, but no melanocytic proliferation - post-inflammatory pigment alteration, ephelis (freckle), labial melanotic macule;
- Pigmented epithelial proliferations - seborrhoeic keratosis, solar lentigo;
- Melanocytic proliferations - simple lentigo, naevi and melanoma.
In the clinical presentation of an atypical pigmented lesion, a biopsy is usually required to distinguish between a melanoma and benign mimics, including dysplastic naevi (see below for information on dysplastic naevi).
Complete excisional biopsies
Elliptical excision and primary closure: complete excision with a 2mm margin is the ideal method for suspicious pigmented skin lesions.
Why is complete elliptical excision the best excision method for these lesions?
- Melanocytic lesions are heterogenous. Diagnostic changes of melanoma may be missed by partial sampling, especially punch biopsy.
- Assessment of size, symmetry and circumscription, required for assessment of melanocytic lesions, is difficult or impossible in a partial sample - especially in the absence of clinical information regarding lesion size and intent of sample (see page 3 for Clinical Information).
- Breslow thickness, which guides management and prognostication, is only accurately determined on examination of the entire intact lesion.
Deep shave excision: also known as ‘saucerisation’.
- Aims to completely remove the lesion at peripheries and in depth.
- May be warranted in low suspicion lesions on trunk and proximal extremities, if palpation does not suggest deeper dermal extension.
- More often associated with positive margins than elliptical excision.
Circumstances where other biopsy types may be appropriate
Broad lesions on the face: shave or incisional biopsy (e.g. lentigo maligna melanoma - see information below).
Large lesions at other sites, functionally sensitive sites (such as the sole of the foot): consider punch or incisional biopsy to confirm diagnosis prior to definitive treatment.
EXCISION MARGINS: WHAT ARE THE RECOMMENDATIONS?
Radial excision margins, measured from the edge of the melanoma, as below:
| pT1 melanoma < 1.0mm | 1 cm |
| pT2 melanoma 1.01 mm - 2.0 mm | 1-2 cm |
| pT3 melanoma 2.01 mm - 4.00 mm | 1-2 cm |
| pT4 melanoma > 4.0 mm | 2 cm |
Depth: down to but not including the deep fascia, unless it is involved or has been reached during the diagnostic excision.
If subcutis is particularly deep, excise to depth equal to the recommended lateral excision margin.
What clinical information should be given to the pathologist evaluating a suspicious pigmented lesion?
- Specimen type, laterality, orientation
- For re-excision specimens: previous laboratory, lab accession number and findings in previous biopsy
- Clinical diagnosis or differential diagnosis
- History of current lesion: duration, duration/tempo of change, size, ulceration
- Any clinically or dermatoscopically suspicious areas?
- History and timing of lesional trauma, biopsy, irritation, treatment with topical agent, laser, radiation therapy
- Past history of melanoma?
- Evidence of current or previous metastatic disease?
- Other relevant history: family history of melanoma or dysplastic naevus syndrome, current or recent pregnancy
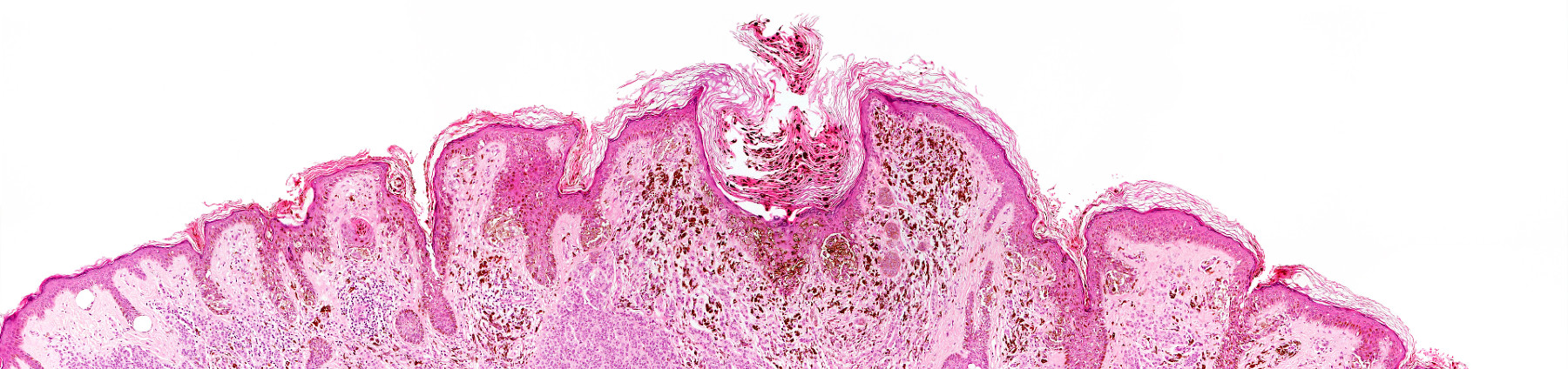
Lentigo maligna melanoma (LMM)
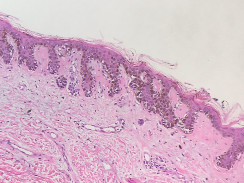
LMM is associated with a high degree of cumulative sun damage, characterised histologically by a lentiginous in situ component called lentigo maligna, also sometimes called LMM in situ.
|
|
|
|
Lentigo maligna – clinical appearance. |
Lentigo maligna – histology. Note prominent solar elastosis, effaced rete and cytologically atypical basal melanocytes. |
These lesions typically occur on the face as a large patch/plaque with mottled areas of different colours. These polymorphous areas represent a mixture and collision of melanocytic and pigmented non-melanocytic lesions, with regression contributing to the overall variegated appearance. Histologically, the melanocytic areas are often variable, comprising an atypical lentiginous melanocytic proliferation that transitions into areas of in situ melanoma or even invasive melanoma. The superimposed nonmelanocytic lesions may include seborrhoeic keratosis, solar lentigo, pigmented solar keratosis and intraepidermal carcinoma.
Partial biopsy of lentigo maligna melanoma is therefore prone to under-diagnosis. Instead, multiple shave biopsies to sample each different area/colour should be performed if complete excision is not feasible.
Shave biopsies are preferred over punches, as width rather than depth is more helpful diagnostically and are more acceptable for healing and cosmesis.
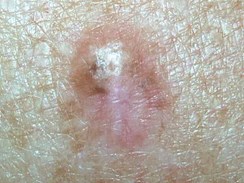
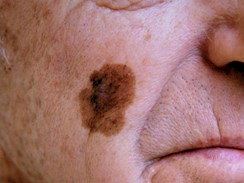
DYSPLASTIC NAEVI
Clinical:
- Atypical
- Macular (flat) component in at least one area and exhibiting at least three of the following features:
- Ill-defined border
- Size at least 5mm
- Colour variegation
- Uneven periphery
- Erythema
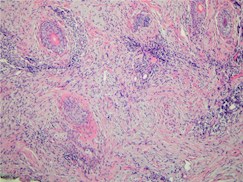
Histology:
- Architectural disorder
- Cytological atypia
|
|
|
|
Dysplastic naevus– clinical appearance. The lesion is more |
Dysplastic naevus – histology. Note the architectural |
Clinical, histologic and genomic features indicate that dysplastic naevi are intermediate between common acquired naevi and in situ melanoma.
The 2018 WHO Classification of Skin Tumours
has removed the entity of ‘mildly dysplastic naevus’ and instead recommends using only two grades of dysplasia: LOW-GRADE and HIGH-GRADE dysplasia.
| WHO Classification (2018) | Former Grade |
| Not a dysplastic naevus | Mild dysplasia |
| Low-grade dysplasia | Moderate dysplasia |
| High-grade dysplasia | Severe dysplasia |
Significance of dysplastic naevi
1. Morphologic mimic of melanoma
2. Potential melanoma precursor
3. Biomarker for increased melanoma risk
To re-excise or not?
Dysplastic naevi are much more common than melanoma and the risk of progression of any given lesion is low. However, for high-grade dysplastic naevi with positive histologic margins, re-excision with a 2-5mm clinical clearance is recommended. If margins are clear but narrowly so (< 2mm), there is currently no clear consensus regarding re-excision.
Low-grade dysplastic naevi
Where there are clear histologic margins and no pigment evident clinically, re-excision is not required, unless there was a high level of clinical concern pre-biopsy. Positive histologic margins: re-excision may not be required, if margins were clinically clear and there is no residual pigment. Observation in this setting remains controversial and is not uniformly accepted clinical practice.
What has become of the formerly known ‘mildly dysplastic naevus’?
These are lentiginous naevi - pigmented lesions that have the appearance of simple lentigo with the addition of one or more small nests of melanocytes. There may be architectural features of dysplastic naevus but these are small lesions with, at most, mild cytological atypia. These lesions are not associated with an increased risk of melanoma and are very common in the general population.
About the author

Dr Jenny Grew
MBChB, FRCPA, AFRACMA
Lab: Subiaco
Areas Of Interest: Breast, gastrointestinal, gynaecological & cutaneous pathology, cytology and molecular pathology
Speciality: Anatomical Pathology
Phone: (08) 9213 2175
Email: jenny.grew@clinicallabs.com.au
Dr Jenny Grew joined Clinical Labs as Clinical Director Anatomical Pathology for WA in 2017. In 2007 Dr Grew moved from New Zealand to Queensland, taking up the role of Pathologist in charge, providing service to 6 public and private hospitals. Jenny is a keen educator and champion of multidisciplinary patient care in private pathology.
Local skin pathologists near you
 Dr Carl Bulliard
Dr Carl Bulliard
BSc (Med), MBBS, Grad Dip Med (Clin Epid), FRCPA
Lab: Bella Vista, NSW
Areas Of Interest: Pathology of the Skin, Head & Neck and Eye, Registrar Training
Speciality: Anatomical Pathology
Phone: (02) 8887 9951
Email: carl.bulliard@clinicallabs.com.au

Dr Irani Dissanayake
MBBS, MD, FRCPA
Lab: Adelaide Airport, SA
Areas Of Interest: Gynaecological Pathology, Gastrointestinal Pathology
Speciality: Anatomical Pathology
Phone: (08) 8205 5641
Email: irani.dissanayake@clinicallabs.com.au

Dr Predrag Nikolic
MBChB, MMedSci, PhD, FRCPA
Lab: Clayton, Vic
Areas Of Interest: Skin Pathology, Lung
Pathology, Breast Pathology, Molecular
Diagnostics
Speciality: Anatomical Pathology
Phone: 1300 134 111
Email: predrag.nikolic@clinicallabs.com.au