- PARTIAL MEDICARE REBATE COMING SOON -
EndoPredict is an in vitro prognostic test that provides highly important and clear information for different stages of treatment planning for patients with estrogen receptor-positive, HER2-negative primary breast cancer.
It helps to define the risk of breast cancer recurrence, the benefits of chemotherapy and who can benefit from extended endocrine therapy.
EndoPredict provides a comprehensive test result and an individualised EPclin Risk Score. The EPclin Risk Score algorithm integrates a 12-gene molecular score, tumour size, and nodal status. All three factors contributed significant information with respect to risk assessment in an independent clinical validation study 1.
In addition to the percentage risk of recurrence up to 10 years, the absolute chemotherapy benefit based on current treatment regimens and the risk of recurrence between 5 and 15 years after diagnosis* is indicated. The patient is classified as “low risk” or “high risk”. The treating physician receives the report and can plan further treatment based on the results.
Under endocrine therapy alone without chemotherapy, more than 95% of low-risk patients do not experience a distant recurrence, even more than 10 years after diagnosis 1. Compared to risk stratification using other gene expression tests or clinical parameters, EndoPredict identifies the largest group of women with breast cancer at low risk (<10% chance of distant recurrence in 10 years) who might safely avoid chemotherapy 2,3,4.
In addition EndoPredict predicts the individual absolute chemotherapy benefit at 10 years 6 and is the only test that provides the individual risk of breast cancer late distant recurrence within years 5-15 to help in deciding whether a patient can avoid extended endocrine therapy. EndoPredict is performed on FFPE tumour tissue from biopsy or surgical specimens. The 12-gene molecular score (also called EP Score in publications) is determined initially.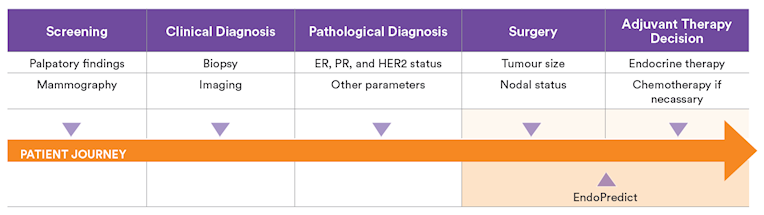
As soon as information on tumour size and nodal status is available, it is combined with the 12-gene molecular score to calculate the EPclin Risk Score.
Molecular Assays in Breast Cancer
Breast cancer patients and their treating doctors must make complex, highly-personalised treatment decisions. Prognostic tools, such as EndoPredict, can play a vital role in determining the type of treatment and prognosis for the patient through assisting with adjuvant therapy decision-making in ER-positive breast cancer.
Studies of gene expression conducted in the early 2000s highlighted the potential of molecular assays to provide additional information beyond traditional pathology regarding prognosis. These assays have all been shown to provide useful prognostic information to ER-positive patients regarding their risk of developing breast cancer recurrence, and their use is supported by international guidelines.
A number of studies have shown that the second-generation assays, such as EndoPredict, which also incorporate clinical variables such as lymph node status and tumour size, are better able to predict late recurrence (5–10 years post treatment) and may also identify a larger group of low-risk patients 3,4.
High quality pathology is a vital part of breast cancer diagnosis and management, and molecular assays such as EndoPredict can provide important additional information to support complex decision-making about the use of chemotherapy in ER-positive breast cancer.
EndoPredict at a Glance
- Only prognostic test that can answer
- whether your patient can safely avoid chemotherapy
- how beneficial chemotherapy would be
- whether your patient can avoid extended endocrine therapy - Largest “true” low risk group for safe reduction of chemotherapy
- more than 70% of N0 patients
- up to 30% of N+ patients - Second-generation gene expression test for superior prognostic power
- Unique gene selection for accurate early and late risk assessment
- Consistent study cohorts and constant cutoff
- Clear low and high-risk category
- Rapid results
Target group check
| ER-Positive | 0-3 pos. Lymph node | Size: pT1-3 |
| HER2/neu-Negative | Pre-/Post-Menopausal |
How to Order: Fill out our Somatic Mutation Testing/EndoPredict request form and tick EndoPredict.
Turnaround Time: 4–5 business days from the sample receipt date.
Specimen Required: EndoPredict is performed on FFPE tumour tissue from biopsy
or surgical specimens.
Test Cost: Partial Medicare rebate coming soon. Currently an out-of-pocket fee of $2980 applies.
For further information, or to enquire about ordering EndoPredict, please contact Australian Clinical Labs Molecular department on (03) 9538 2259.
References:
1. Filipits M et al. (2011) Clin Cancer Res, 17(18):6012–6020.
2. Dubsky P. et al. (2013) Ann Oncol, 24:640–647.
3. Buus et al. (2016) J Natl Cancer Inst, 108:11pp.
4. Sestak I et al. (2018) JAM Oncology Published online February 15, 2018.
5. Martin M et al.: BCR, 16:R3.
6. Sestak I et al. (2018) SABCS 2018.
7. Filipits M et al. (2018) SABCS 2018.
8. Dubsky P et al.: The EndoPredict score provides prognostic information on late distant metastases in ER+/HER2- breast cancer patients. BJC.
Download EndoPredict Expanded Brochure Download EndoPredict Summary Brochure
Download EndoPredict Request FormDownload EndoPredict Request Form (WA)
Download EndoPredict Patient Brochure
Somatic Mutation | Solid Tissue Molecular Profiling in Breast Cancer
In breast cancer, oncogenic mutations in PIK3CA or ERBB2 amplification (along with TP53 mutations) occur in ~25% of cases1,2. As such, mutated PI3K has become an attractive therapeutic target in breast cancer therapy, and a number of agents targeting the PI3K pathway are currently in clinical development3.
Genes sequenced in this panel include:
| PIK3CA | TP53 |
| AKT1 | PTEN |
| ERBB2 |
When to Order: At diagnosis.
How to Order: Fill out our Somatic Mutation testing request form and tick the Somatic Mutation test panel required.
Turnaround Time: 5–7 business days from the sample receipt date.
Specimen Required: Fresh formalin-fixed paraffin-embedded (FFPE) of 5-10 μm thickness from the tumour tissue.
Test Cost: No Medicare rebate available. An out-of-pocket fee of $400 applies.
A negative result does not rule out the presence of a mutation that may be present but below the limits of detection for this assay (<1%).
References:
1. Lee JW, et al., (2005) Oncogene 24(8):1477–1480.
2. Levine DA, (2005) Clin Cancer Res 11(8):2875–2878.
3. Malone E et al., Genome Medicine (2020). Molecular profiling for precision cancer therapies 12:8.




