By Dr Stella Pendle
In July 2022, the World Health Organization (WHO) declared monkeypox (now called mpox) a public health emergency of international concern and called for a coordinated response to slow the spread of the disease. Since then, mpox has been raging in Africa but slowly simmering in the rest of the world. In mid-August 2024, the first case of clade Ib, a new clade of mpox, was reported in Sweden from a patient who had travelled to Africa. On August 14th, the WHO announced its second public health emergency of international concern relating to mpox.
Mpox is spreading in Australia; there have been 63 cases notified in NSW since mid-June, with transmission occurring in both regional NSW and Sydney. Victoria has reported 120 mpox cases since April 2024. The new clade 1b has not been reported in Australia yet.
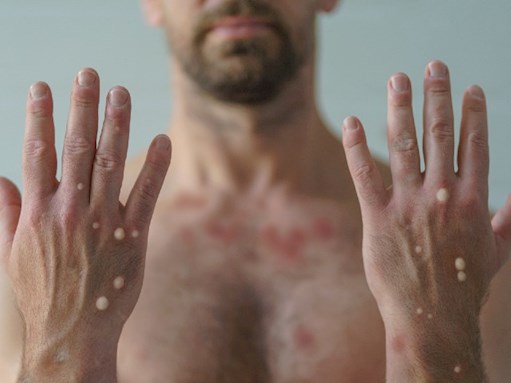
Figure 1. Raised vesicles on the hands of a male with mpox.
The mpox virus was first discovered in 1958, causing a pox-like disease in laboratory monkeys. The first case of human infection was reported in 1970 in a 9-month-old boy in the Congo. Mpox is an enveloped double-stranded DNA virus that belongs to the Orthopoxvirus genus of the Poxviridae family and is closely related to smallpox. The mpox virus has two distinct genetic clades: clade one (I), previously known as the Congo Basin clade, and clade two (II), previously the West African clade. The Congo Basin clade has historically caused more severe disease and was thought to be more transmissible.
How is mpox spread?
Mpox is transmitted by direct contact via respiratory droplets or exposure to infectious lesions on the skin or other bodily fluids. It may be acquired during close sexual contact. Contact with materials used by an infected person, including clothing or bedding, may also lead to transmission. It is unknown if the virus can be transmitted by individuals without skin lesions, and there is no evidence that it is spread by casual contact. Perinatal transmission can occur, leading to congenital mpox.
Clinical features
To date, most cases have occurred in MSM, but there have also been reports overseas of women and children acquiring the infection. The mean incubation period from the time of exposure to the first symptom appearing is 7 days, with 95% of individuals developing symptoms within 17 days.
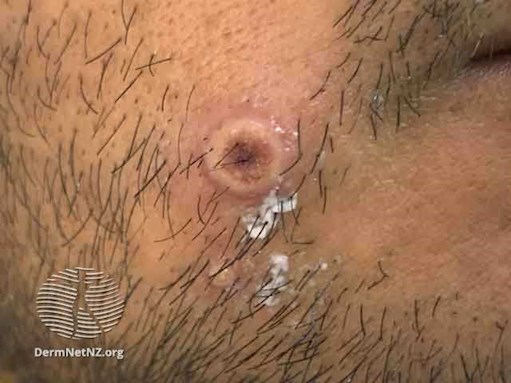
Figure 2. Umbilicated mpox vesicle on male’s cheek at day 4 of infection.
Initial symptoms include a flu-like illness with fever, malaise, headache and fatigue. This is often accompanied by lymphadenopathy. Shortly after the prodrome, a rash appears with lesions starting as macules and progressing to raised papules and vesicles (see Figures 1 & 2). The vesicles may fill with pus, ulcerate, then scab and fall off. The rash is typically distributed on the face, extremities and genitals. MSM may experience symptoms that include anorectal pain, proctitis with bleeding, penal oedema with balanitis and phimosis. Sore throat, odynophagia, epiglottitis and tonsillitis may also occur. The most common location of lesions reported in MSM were the anogenital area (73%), trunk and extremities (55%), face (25%) and palms and soles (10%). Most cases had fewer than 10 lesions, and some had only a single genital lesion.
Complications can occur in immunosuppressed individuals, pregnant women and young children. These include pneumonia, encephalitis and eye infections. Hospitalisation is uncommon and mortality rare, but at least four people in nonendemic countries have died. People should remain in isolation for the duration of the illness, which usually lasts 2 to 4 weeks. The disease is notifiable in Australia.
Like many viruses, mpox cannot be diagnosed by symptoms alone. The symptoms closely resemble those of other rash-producing illnesses such as chickenpox, zoster, measles, syphilis, scabies, bacterial skin infections and allergic reactions. Laboratory testing is, therefore, essential for an accurate diagnosis.
Treatment
For most patients, management is symptomatic, including pain relief. Treatment with antivirals is recommended for people with severe disease or who are at high risk of severe disease.
Vaccination
Vaccination is available in Australia for high-risk groups from the Department of Health. The JYNNEOS vaccine is FDA approved for smallpox and mpox. It uses live attenuated vaccinia virus that is incapable of replicating. It is administered as a two-dose series, with peak antibody response occurring 2 weeks after the second dose. It is thought to be 85% effective at preventing mpox. The vaccine can be administered as post-exposure prophylaxis. When administered up to 4 days after exposure, vaccination can prevent disease onset altogether, but even receipt of vaccine up to 2 weeks after exposure can reduce symptom severity.
Prevention of Infection
The risk of transmission of mpox in the healthcare setting is low if appropriate personal protective equipment is worn. Healthcare workers should wear a gown, gloves, eye protection and an N95 mask. A person with suspected or confirmed mpox infection should be masked immediately, have lesions covered and be placed in a single-person room.
A person with mpox infection should avoid close contact with others until the lesions are completely healed. This can take several weeks. It is unknown whether recovery from mpox protects against subsequent infection.
If you enjoyed this article, subscribe to our electronic Pathology Focus newsletter.
How to Order mpox Testing
Patients at risk of acquiring mpox should be tested. Therefore, obtaining a detailed clinical history, including travel and lifestyle, is essential.
Any unusual skin lesions should be investigated, particularly in the anogenital area. The rash may be limited to only a few lesions or even a single lesion.
Complete the Clinical Labs General Pathology Request Form, including mpox and other tests for investigation.
Nucleic acid amplification testing, also known as polymerase chain reaction (PCR), is the recommended test for diagnosis of mpox.
The WHO recommends the collection of fluid samples from pustules, dried crusts or scabbed lesions using a plain dry, sterile swab suitable for PCR testing.
At least 2 swabs should be collected from 2 different sites, if possible, to improve uptake.
The swabs for mpox should be packed separately to assist the laboratory in processing the specimens promptly.
These are referred to the local public health laboratory for testing.
If clinically indicated, testing for other likely pathogens should also be performed, including herpes simplex, varicella zoster or bacterial infection.
Antibody and antigen testing currently has limited utility due to cross-reactions with other Orthopoxviruses and is not recommended.
References
- Update on the Monkeypox Outbreak. Del Rio C, Malani, PN. JAMA published online August 11, 2022. doi:10.1001/jama.2022.14857
- Monkeypox from WHO - https://www.who.int/news-room/fact-sheets/detail/monkeypox
- Australian Government monkeypox resources - https://www.health.gov.au/resources/collections/monkeypox-mpox-resources
- Victorian Government Update - https://www.health.vic.gov.au/health-alerts/mpox-cases-on-the-rise
- NSW Government Update - https://www.health.nsw.gov.au/Infectious/alerts/Documents/2024/20240802-gp-alert-mpox.pdf
Figure 2 reproduced with permission from ©DermNet www.dermnetnz.org 2022.