By Associate Professor Mirette Saad
Pharmacogenetics (PGx), an important part of precision medicine, is the study of how genetic variability influences drug treatment outcomes. Recommended by Guidelines, many medications currently prescribed have pharmacogenetic data to support appropriate dosing or selection. Like all diagnostic tests, pharmacotherapeutic genotyping is one of multiple pieces of information that clinicians should consider when making their therapeutic choice for each patient.
Clinical Labs offers a comprehensive range of pharmacogenetic testing in order to provide Clinicians and Healthcare providers with important information to help decide on the most appropriate treatment for each individual, particularly in areas such as mental health, pain management, cardiology and oncology.
"The field of pharmacogenetics involves
|
PGx test utility
Implementation of clinical pharmacogenetics, allele function and inferred phenotypes is a crucial step toward optimum patients’ health. Identifying responders and nonresponders to medications can reduce morbidity, avoid adverse events and optimise drug dosing.
Literature has shown that a large number of people are injured or die each year in hospitals from adverse drug events (ADEs), costing millions of dollars in healthcare costs each year. The field of genomic medicine presents one potential solution to reduce healthcare costs associated with ADEs and poor response to pharmacotherapy.
PGx guidelines
Evidence-based guidelines with pharmacotherapeutic recommendations for combinations of specific drugs and genotypes or predicted phenotypes are essential for implementing acquired pharmacogenetics knowledge in daily clinical practice.
The Dutch Pharmacogenetics Working Group (DPWG) and the Clinical Pharmacogenetics Implementation Consortium (CPIC) have been developing guidelines for more than a decade (Swen et al. 2011a; Caudle et al. 2017).
Recommendations are preferably made available at the time of drug prescribing and dispensing for a patient with a genotype that requires an action, such as a dose reduction (Swen et al. 2011a; Deneer and van Schaik, 2013).
When to order the test?
Physicians may order the pharmacogenetic testing per drug at the point of care, or an alternative approach to ordering is the use of pre-emptive testing, perhaps as part of an annual exam in young adults or even children that require multiple treatments. As a result of the increasing number of drugs with pharmacogenetic data, the pre-emptive use of testing could significantly optimise drug outcomes (Schildcrout et al. 2012).
Regardless of when ordered (at time of treatment or prior), due to the continuing decline in the costs of genomic testing technologies, a broad-based pharmacogenetic screen may yield the greatest cost savings.
The cytochrome P450 (CYP450) and differences in drug metabolism
A family of enzymes (Figure 1), catalyses the metabolism of many drugs and xenobiotics (Table 1). The genes that code for cytochrome P450 enzymes are highly polymorphic, which can significantly affect drug metabolism in certain individuals. Differences in drug metabolism due to CYP450 gene variants influence plasma levels of both the active drug and its metabolites.
For example, CYP2D6, CYP2C19 and CYP2C9 are responsible for the metabolism of a large number of commonly prescribed drugs, including warfarin, analgesics, clopidogrel, codeine, tamoxifen, some antidepressants, statins, proton pump inhibitors (PPIs) and anti-emetics (See Table 1). CYP3A5 genotype results can be used to guide dosing of tacrolimus in organ transplant patients (Birdwell et al. 2015).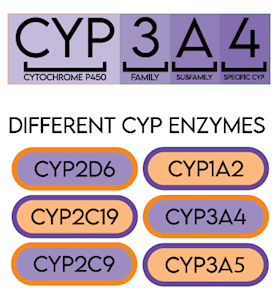
Figure 1. CYP Cytochrome P450 enzyme nomenclature and examples chart.
CYP2D6
CYP2D6 is the primary enzyme responsible for the metabolism of many commonly-used medications especially in mental health, oncology (tamoxifen and 5-HT3 receptor antagonists) (Goetz et al. 2018) and pain management (Crews et al. 2021) (Table 1 & Figure 2). CYP2D6 is highly polymorphic with over 130 identified allelic variants and sub-variants identified (www.PharmVar.org; CYP2D6 Allele Definition).
CYP2D6 alleles have been extensively studied in multiple geographically, racially, and ethnically diverse groups, and significant differences in allele frequencies have been observed. It is important to note that variation in CYP2D6 may have implications for many therapies that may not be listed on this report (Gaedigk et al. 2017).
Figure 2. Cytochrome P450 (CYP2D6) liver enzyme in complex with a drug.
CYP2C19
The hepatic CYP2C19 enzyme contributes to the metabolism of a large number of clinically relevant drugs such as antidepressants, benzodiazepines, mephenytoin, some proton pump inhibitors (Lima et al. 2021), and clopidogrel (Scott et al. 2013) and anti-fungal medication (voriconazole) (Moriyama et al. 2017) (Table 1). Like many other CYP450 superfamily members, the CYP2C19 gene is highly polymorphic, with >25 known variant alleles.
CYP2C9
Variants in the CYP2C9 genes modify the rate at which some medications are metabolised. When considering antidepressant therapy such as tricyclic anti-depressants (TCAs), CYP2C9 test is often combined with analysis of the CYP2C19 and CYP2D6 genes (Attia et al. 2014 and Hicks et al. 2017). When considering warfarin therapy, this test is often combined with analysis of VKORC1.
VKORC1 and CYP2C9 and warfarin
Warfarin is one of the most commonly prescribed medications worldwide, used for many indications including prophylaxis and treatment of thromboembolic disorders, atrial fibrillation, or cardiac valve replacement, and systemic embolism after myocardial infarction (MI). Approved in the US in 1954, the high efficacy of warfarin is challenged by the high risk of ADEs due to its narrow therapeutic window, requiring careful monitoring and strict
compliance.
While CYP2C9 is predominantly involved in the metabolism of warfarin subtypes; VKORC1 is the molecular target of the drug. In 2017, an international collaboration published an updated landmark paper defining appropriate warfarin doses based on a validated dosing algorithm of clinical biomarkers and VKORC1/CYP2C9 genotypes (Johnson et al. 2017).
SLCO1B1 and statins
SLCO1B1 gene testing is clinically important in clearance of statins, especially simvastatin. Myopathy is reported in poor metabolisers of this gene. Alternative lipid lowering statins can be prescribed in lower doses such as atorvastatin, pravastatin and rosuvastatin (Ramsey et al. 2014).
Genetic variations can render some medications ineffective or toxic
Pharmacogenetic variants result in four distinct phenotypes: normal metabolisers (NMs), intermediate metabolisers (IMs), poor metabolisers (PMs), and ultrarapid metabolisers (UMs) which provides guidance to drug dosing and selection.
Overall, wild-type alleles are usually associated with functional enzyme-mediated metabolism. Ultrarapid metabolisers may not achieve therapeutic plasma levels due to decreased trough drug concentrations, whereas poor metabolisers treated with drugs that are metabolised by these enzymes are at increased risk for prolonged therapeutic effect or toxicity due to increased trough levels of therapeutic drugs.
Some anti-psychotic and SSRI medications can be contraindicated in intermediate CYP2D6 metabolisers due to increased risk of adverse effects and so alternative agents must be prescribed.
CYP2D6 ultrarapid metabolisers treated with codeine exhibit symptoms of extreme sleepiness, confusion or shallow breathing; the lowest possible dose should be prescribed to these patients. Meanwhile, patients that are CYP2D6 poor metabolisers will not achieve sufficient pain control due to their inability to convert the drug to its active form of morphine (Crews et al. 2021).
CYP2C19 ultrarapid metabolisers should be prescribed alternative therapeutic agents other than benzodiazepines, such as citalopram (Celexa) and escitalopram (Lexapro) and TCAs such as impiramine (Tofranil) and clomipramine (Anafranil) due to possible decreases in the efficacy of these medications.
Pharmacogenetic markers in oncology
In addition to RAS, BRAF, EGFR, ERBB2 (HER2), PK3CA and KIT mutation and PD-1, ROS, ALK and BCR-ABL fusion genes, other genetic pharmacogenetic biomarkers play a role in patients’ responses to oncology therapy.
UDP-glucuronosyltransferase gene (UGT1A1)
UGT1A1 is involved in the metabolism of irinotecan (Figure 3), a topoisomerase I inhibitor. UGT1A1 gene polymorphism is associated with toxicity and clinical efficacy of irinotecan-based chemotherapy in patients with advanced solid tumours including colorectal, rectal and lung cancer (Fujii et al. 2019).
Figure 3. Irinotecan cancer chemotherapy drug molecule.
Thiopurine methyltransferase (TPMT)
TPMT is the primary enzyme responsible for thiopurine drugs (azathioprine, 6-mercaptopurine and 6-thioguanine) metabolism. These drugs are converted in the body to thioguanine nucleotides (TGNs).
Thiopurine therapy targets the replicating cells without overly harming normal cells. Several studies have established Single Nucleotide Polymorphisms (SNPs) in the TPMT gene that may lead to enzyme inactivity and therefore haematopoietic toxicity due to thiopurine drugs. It is recommended that physicians order TPMT genotyping before prescribing thiopurines to avoid bone marrow toxicity and consequent neutropenia (Relling et al. 2018).
Dihydropyrimidine dehydrogenase gene (DPYD)
DPD stands for dihydropyrimidine dehydrogenase, an enzyme made by the liver that breaks down uracil and thymine. The molecules created when pyrimidines are broken down (5,6-dihydrouracil and 5,6-dihydrothymine) are excreted by the body or used in other cellular processes. DPYD gene mutations result in excess quantities of the breakdown molecules in the blood, urine, and cerebrospinal fluid.
Mutations in the DPYD gene also interfere with the breakdown of drugs with structures similar to the pyrimidines, such as the cancer drugs 5-fluorouracil and capecitabine (two common chemotherapy drugs used as a treatment for a number of different cancers). As a result, these drugs accumulate in the body and cause the severe reactions and neurological manifestations as a result of DPD deficiency (Amstutz et al. 2017).
Conclusion
The incorporation of genetic information obtained from pharmacogenetic testing holds substantial promise to improve therapeutic decision making through improved efficacy and reduced adverse events. Considerations for clinical implementation, such as optimal laboratory workflows, electronic health record integration, and stakeholder engagement, as well as provider education, are crucial for patients’ health.
Pharmacogenetic (PGx) test list at Australian Clinical Labs
Our comprehensive pharmacogenetic tests can detect polymorphisms in genes coding for drug metabolising enzymes that predispose individuals to metabolising drugs inadequately.
Gene panels offered:
• Cytochrome P450 Comprehensive Gene Panel including*:
CYP2D6, CYP2C9, CYP2C19, CYP3A4, CYP3A5, CYP1A2, SLCO1B1 and VKORC1
Single gene tests:
• TPMT (Medicare rebate)
• DPYD
• UGT1A1
• CYP2D6
• CYPC9
• CY2C19
*Please note that the panel Cytochrome P450 Genes can be ordered separately or together (CYP2D6, CYP2C9, CYP2C19, CYP3A4, CYP3A5, CYP1A2, SLCO1B1 and VKORC1).
For a full listing of genes tested and drugs metabolised, see the table below.
Please note that this is a guide for gene selection. Some specific medications may not be reported if they are listed under a drug class that is metabolised by the relevant gene.
Table 1: Selection guide of genes tested based on medications of use
| GENE | Type of Metabolised Medication | Metabolised DRUG | Medicare Rebate | |
|
CYP2D6 |
|
Anti-Psychotics |
Opioids and Pain Management Codeine (prodrug) Dihydrocodeine Morphine Naltrexone Oxycodone Tramadol Neurology, Anti-ADHD and Anti-Dementia Atomoxetine Dextroamphetamine Donepezil Galantamine Lisdexamfetamine Urology Medication Darifenacin Mirabegron Tamsulosin Tolterodine Others Metoclopramide Ondansetron |
N/A |
| CYP2C9 |
|
NSAIDs Celecoxib Flurbiprofen Ibuprofen Meloxicam Piroxicam |
Neurology Phenytoin Anti-Coagulant Warfarin |
N/A |
| CYP2C19 |
|
PPIs: |
TCAs |
N/A |
| SLCO1B1 |
|
Pravastatin Simvastatin |
N/A | |
| CYP1A2 |
|
Clozapine Duloxetine Olanzapine |
|
N/A |
| CYP3A4 |
|
Atorvastatin Codeine Diazepam Quetiapine Simvastatin Tacrolimus |
N/A | |
| CYP3A5 (and CYP3A4) |
|
Tacrolimus | N/A | |
| VKORC1 |
|
Warfarin | N/A | |
| TPMT |
|
Azathioprine Cisplatin Mercaptopurine Thioguanine |
YES | |
| DPYD |
|
Capecitabine |
N/A | |
| UGTA1 |
|
Atazanavir |
N/A | |
Content in Table 1 is correct as of 16.05.23.
|
When to order: How to order: Turnaround time: Specimen required: Report: Test cost: |
References:
Amstutz et al. 2017. Clin Pharmacol Ther
Attia et al. 2014. Chem Pharm Bull
Birdwell et al. 2015. Clin Pharmacol Ther
Caudle et al. 2017. Clin Pharmacol Ther
Crews et al. 2021. Clin Pharmacol Ther
Deneer and van Schaik, 2013. Pharmacogenomics
Fujii et al. 2019. Cancer Chemotherapy and Pharmacology
Gaedigk et al. 2017. Clin Pharmacol Ther
Goetz et al. 2018. Clin Pharmacol Ther
Guidelines – CPIC (cpicpgx.org)
Hicks et al. 2017. Clin Pharmacol Ther
Johnson et al. 2017. Clin Pharmacol Ther
Klein et al. 2009. N Engl J Med
Lima et al. 2021. Clin Pharmacol Ther
Moriyama et al. 2017. Clin Pharmacol Ther
Ramsey et al. 2014. Clin Pharmacol Ther
Relling et al. 2018. Clin Pharmacol Ther
Schildcrout et al. 2012. Clin Pharmacol Ther
Scott et al. 2013. Clin Pharmacol Ther
Swen et al. 2011a. Clin Pharmacol Ther
About the author:
Assoc. Prof. Mirette Saad
National Director of Molecular Genetics
Australian Clinical Labs
MBBS (HONS), MD (HONS), MAACB, FRCPA, PHD
Lab: Clayton
Speciality: Chemical Pathology
Areas of Interest: Molecular genetics, precision medicine, cancer genetics, antenatal screening, NIPT, endocrine, fertility testing and research, medical teaching
Phone: (03) 9538 6777
Email: mirette.saad@clinicallabs.com.au
Associate Professor Mirette Saad is a Consultant Chemical Pathologist and the National Director of Molecular Genetics at Australian Clinical Labs. At Clinical Labs, A/Prof Mirette Saad leads the Molecular Genetic testing for non-invasive prenatal testing (NIPT), antenatal screening, personalised drug therapy and cancer. She is a Chair of the RCPA Chemical Pathology Advisory Committee, Member of the RCPA Genetic Advisory Committee, AACB and a Chair of the Precision Medicine Services at Australian Clinical Labs.




