Medicare Benefits Schedule ChangesFrom 1 November 2021, certain patients may be eligible for a Medicare rebate. Medicare Item information below:
|
By Associate Professor Louise Smyth
Faecal calprotectin concentration is a useful, non-invasive method for distinguishing between organic and functional diarrhoeal disorders and for monitoring disease activity in established cases of gut wall inflammation.
Calprotectin concentrations are assayed on the DiaSorin Liaison XL instrument using chemiluminescent immunoassay technology. Reference intervals (RIs) are provided for adult patients. A large body of literature exists that supports the use of faecal calprotectin concentration in children, infants and neonates, as in adults. RIs in paediatric populations are poorly established but published faecal calprotectin concentrations, in health, are higher than in adults.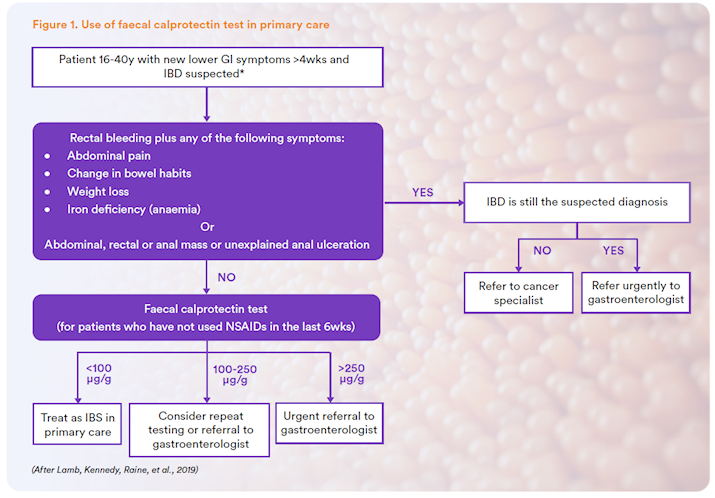
Calprotectin
Calprotectin is a large protein with post-translational changes that include binding of each of zinc and calcium at different sites.
Although described in multiple cell types, the protein is generally used as a marker of neutrophilic inflammation. Calprotectin accounts for 60% of the protein content of the cytosol of neutrophils (Roseth, Fagerhol MK, Aadland, & Schjonsby, 1992). Actions of calprotectin include antimicrobial and anti-proliferative activity and an extracellular immunomodulatory effect. The name calprotectin was adopted because of its calcium binding properties and protective functions (Johne, Fagerhol, Lyberg, Prydz, & Brandt, 1997). As it is a member of the S100 protein family, it is officially designated S100 Calcium-Binding Protein A8 (HGNC Approved Gene Symbol: S100A8) (McKusick & Converse, 2015).
Clinical uses of calprotectin
Calprotectin concentrations have been shown to reflect pathology in many tissues and in associated body fluids, including plasma, saliva, urine and synovial fluid and there is an increasing literature referencing its assay in multiple clinical conditions.
Faecal calprotectin
Widespread clinical application of measurement of calprotectin levels has evolved for the non-invasive
investigation of diarrhoeal gut disease, since the beginning of this century. Early faecal assays used stool volume vs. weight and the reported measurement units and RIs of these assays, therefore, differ from modern methods. Calprotectin in faeces has been shown to be a robust measure of neutrophilic inflammation of the intestinal mucosa. However, it is not specific for inflammatory bowel disease (IBD), being variably increased in other causes of gut wall inflammation and in various gastrointestinal malignancies and ingestion of some common drugs. Nevertheless, faecal calprotectin concentration has been shown to vary with the degree of inflammation (Bressler, Panaccione, Fedorak, & Seidman, 2015) and by some authors to predict relapse in IBD (Chang, Malter, & Hudesman, 2015).
Table 1 - Factors and conditions associated with elevated faecal calprotectin levels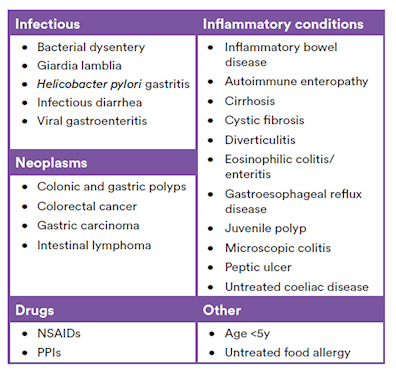
NSAIDs (Nonsteroidal anti-inflammatory drugs); PPIs (Proton pump inhibitors)
(After Bressler, Panaccione, Fedorak, & Seidman, 2015)
Although faecal calprotectin concentration increases somewhat with age in adults, it has been shown to be a superior non-invasive discriminatory test for the distinction between inflammatory and functional intestinal disease (Figure 2). PPV is increased by a raised, concomitant serum CRP.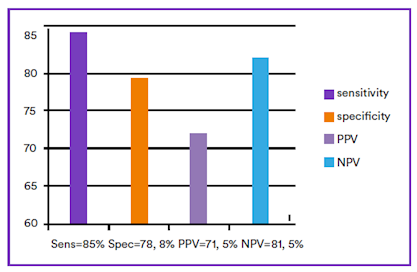
Figure 2. Pooled faecal calprotectin sensitivities, specificities, positive predictive value and negative predictive value of faecal calprotectin in discriminating between intestinal inflammation and functional disorders (Mumolo, et al., 2018). PPV: Positive predictive value; NPV: Negative predictive value.
Faecal calprotectin in paediatric populations
Several authors have established the usefulness of faecal calprotectin concentration in children. However, it is known that young children and infants have higher faecal calprotectin concentration than adults or older children. The literature regarding older children is less clear regarding RIs; however, the longitudinal monitoring of individuals is clinically reliable (Herrera, Christensen, & Helms, 2016). Neonates and premature infants appear to have complicated faecal calprotectin concentration responses, falling immediately after birth and then rising.
Li et al. (2015) have published faecal calprotectin levels from 288 healthy children 0-18 months. Their data is shown below (Figure 3).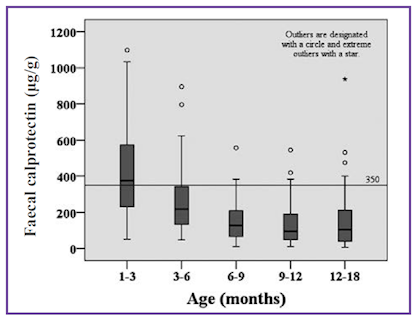
Figure 3. Faecal calprotectin concentrations in five age groups of healthy children (Li, et al., 2015).
The same group have also published equivalent data for 274 children 1 to 4 years (Figure 4).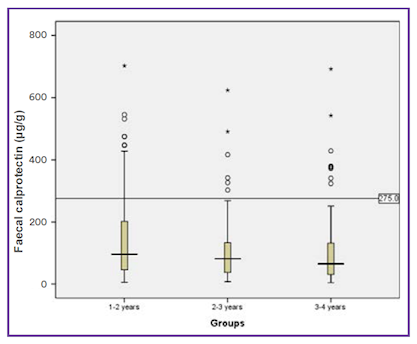
Figure 4. Faecal calprotectin concentrations in three age groups of healthy children (Zhu, Li, Wang, Shen, & Sheng, 2016).
Necrotising enterocolitis is associated with increased faecal calprotectin concentration. Wide ranging cut-offs are reported however, Thuijls et al. (2010) have reported clinically relevant positive likelihood ratio (LR) of 12.29 and negative LR of 0.15 using a faecal calprotectin concentration cut-off of 286.2 μg/g faeces.
Laboratory analysis of faecal calprotectin
Reference intervals
Most healthy adults will have a faecal calprotectin concentration <10 μg/g faeces. However, as the faecal calprotectin concentration is known to increase with age, RIs are usually established that account for miscellaneous factors, including age. These RIs should not be used for longitudinal monitoring when the patient should become their own reference.
Based on the expected values from literature reports and the manufacturer’s recommendation, Clinical Labs has adopted the following RIs for faecal calprotectin concentration in adult patients with clinically suspected IBD:
| 0-50 μg/gram | IBD unlikely but not excluded. |
| 50-100 μg/gram | IBD likely; other inflammatory conditions, including but not limited to infection, coeliac disease and diverticular disease, cannot be excluded. |
| 100 μg/gram | Almost exclusively IBD. Other severe inflammatory diseases not excluded. |
Conclusion
Faecal calprotectin concentration is a safe and reliable non-invasive test for inflammation of the bowel wall that can:
- Distinguish between patients with IBD and patients with IBS.
- Determine disease activity and risk of relapse in IBD patients, and assess the level of mucosal healing.
- Help to identify patients with abdominal symptoms who may require further investigative procedures and reduce the number of endoscopies performed for the diagnosis of diarrhoeal disease and monitoring of IBD.
Because it is not specific for IBD, it must be interpreted in the clinical context.
Specimen collection, transport and storage
The time between defaecation might affect the faecal calprotectin concentration; therefore, the first stool of the day is recommended. Stool specimens should be collected into a clean, airtight container without preservative and stored at 2-8°C. The sample must be received at the laboratory within 24hrs of collection. Stool specimens that are liquid or very solid may be technically unsuitable.
Turn-around time
Results are available within 3 to 4 business days of the specimen’s arrival at our laboratory.
References
- Bressler, B., Panaccione, R., Fedorak, R. N., & Seidman, E. G. (2015). Clinicians’ guide to the use of fecal calprotectin to identify and monitor disease activity in inflammatory bowel disease. Can J Gastroenterol Hepatol., 29(7), 369-372.
- Chang, S., Malter, L., & Hudesman, D. (2015, October 28). Disease monitoring in inflammatory bowel disease. World J Gastroenterol, 21(40), 11246-11259.
- Herrera, O. R., Christensen, M. L., & Helms, R. A. (2016). Calprotectin: Clinical Applications in Pediatrics. J Pediatr Pharmacol Ther, 21(4), 308-321.
- Johne, B., Fagerhol, M. K., Lyberg, T., Prydz, H., & Brandt, P. (1997). Functional and clinical aspects of the myelomonocyte protein calprotectin. J Clin Pathol:Mol Pathol, 50, 113-123.
- Lamb, C. A., Kennedy, N. A., Raine, T., & et al. (2019, September 27). British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut, 68, s1-s106. Retrieved from http://dx. doi. org/ 10. 1136/gutjnl-2019-318484
- Li, F., Ma, J., Geng, S., Wang, J., Liu, J., Zhang, J., et al. (2015). Fecal Calprotectin Concentrations in Healthy Children Aged 1-18 Months. PLoSONE, 10(3).
- McKusick, V. A., & Converse, P. J. (2015, July 23). S100 CALCIUMBINDINGPROTEIN A8; S100A8. Retrieved December 8, 2016, from Online Mendelian Inheritance in Man: https://www.omim.org/entry/123885?search=calprotectin&highlight=calprotectin
- Mumolo, M.G., Bertani, L., Ceccarelli, L., Laino, G., Di Fluri, G.,Albano, E., Tapete, G., Costa, F. (2018). From bench to bedside: Fecal calprotectin in inflammatory bowel diseases clinical setting. World J Gastroenterology, 24 (33):3681-3694.
- Roseth, A. G., Fagerhol MK, M. K., Aadland, E., & Schjonsby, H. (1992). Assessment of the neutrophil dominating protein calprotectin in feces. A methodologic study. Scand J Gastroenterol, 27, 793-798.
- Thuijls, G., Derikx, J. P., van Wijck, K., Zimmermann, L. J., Degraeuwe, P. L., & Mulder, T. L. (2010). Non-invasive markers for early diagnosis and determination of the severity of necrotizing enterocolitis. Annals of Surgery, 251, 1174-1180.
- Zhu , Q., Li, F., Wang , J., Shen, L., & Sheng, X. (2016). Fecal Calprotectin in Healthy Children Aged 1-4 Years. PLoS ONE, 11(3).
About the author:
 Assoc. Prof. Louise Smyth
Assoc. Prof. Louise Smyth
BA MBBS GCUT DipHPE FRCPA
Lab: Osborne Park
Speciality: Allergy and Immunology
Areas of Interest: Autoimmunity, transplantation, immune deficiency, allergy
Phone: 1300 367 674
Email: Louise.Smyth@clinicallabs.com.au
Associate Professor Smyth is a graduate of the University of Western Australia and a Fellow of, and former state representative of the RCPA. Associate Professor Smyth designed and implemented the Pathology programme for the School of Medicine at the University of Notre Dame Australia, Fremantle where she is a founding member of, and Associate Professor in the School of Medicine. She has a Graduate Certificate in University Teaching, qualifying her to supervise candidates for higher degrees as well as teaching undergraduate students. She is most interested in autoimmunity but has extensive experience including autoimmunity, transplantation, immune deficiency and allergy. Her publications are predominantly in the field of Bone Marrow Transplantation. Dr Smyth joined St John of God Pathology (now Australian Clinical Labs) in 2016.